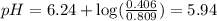Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3



Chemistry, 23.06.2019 06:30, kaitlynk0
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
What is the pH of a buffer prepared by adding 0.809 mol of the weak acid HA to 0.406 mol of NaA in 2...
Questions in other subjects:


Mathematics, 23.04.2021 20:40

Spanish, 23.04.2021 20:40

Mathematics, 23.04.2021 20:40

English, 23.04.2021 20:40



Mathematics, 23.04.2021 20:40

Mathematics, 23.04.2021 20:40

Social Studies, 23.04.2021 20:40

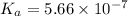
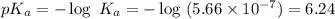
![ pH=pK_a+log\frac{[salt]}{acid]} ](/tpl/images/0539/5199/2cb89.png)