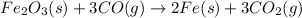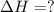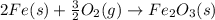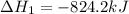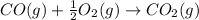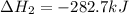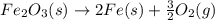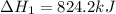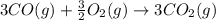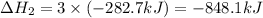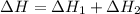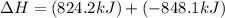Chemistry, 10.03.2020 00:25 skylar7192
Calculate ΔHrxn for the following reaction: Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g) Use the following reactions and given ΔH values: 2Fe(s)+32O2(g)→Fe2O3(s),ΔH CO(g)+12O2(g)→CO2(g),ΔH==−824.2kJ−2 82.7kJ

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, browndalton55
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1



Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1
You know the right answer?
Calculate ΔHrxn for the following reaction: Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g) Use the following reactio...
Questions in other subjects:

History, 01.02.2021 20:40

Arts, 01.02.2021 20:40


Mathematics, 01.02.2021 20:40

English, 01.02.2021 20:40

Mathematics, 01.02.2021 20:40

Chemistry, 01.02.2021 20:40

Mathematics, 01.02.2021 20:40


English, 01.02.2021 20:40

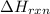 is, -23.9 kJ
is, -23.9 kJ