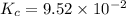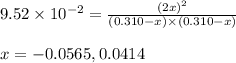He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) + CCl4(g)→ 2CH2Cl2(g)
Calculate the equilibrium concentrations of reactants and product when 0.310 moles of CH4 and 0.310 moles of CCl4 are introduced into a 1.00 L vessel at 350 K.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) +...
CH4(g) +...
Questions in other subjects:


Spanish, 25.11.2021 14:00




Physics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00

History, 25.11.2021 14:00


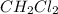 are 0.2686 M, 0.2686 M and 0.0828 M respectively.
are 0.2686 M, 0.2686 M and 0.0828 M respectively.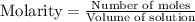
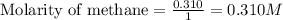
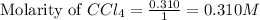
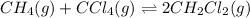
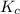 for above equation follows:
for above equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0539/2756/bf52a.png)