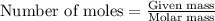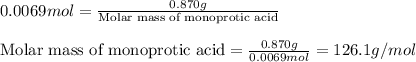Chemistry, 10.03.2020 00:50 kylediedrich1343
A 0.870 g sample of a monoprotic acid is dissolved in water and titrated with 0.300 M KOH.
What is the molar mass of the acid if 23.0 mL of the KOH solution is required to neutralize the sample?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1


Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
A 0.870 g sample of a monoprotic acid is dissolved in water and titrated with 0.300 M KOH.
Wha...
Wha...
Questions in other subjects:

World Languages, 18.04.2020 02:43





Mathematics, 18.04.2020 02:43




Computers and Technology, 18.04.2020 02:43

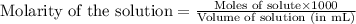

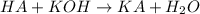
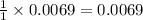 moles of HA
moles of HA