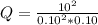Chemistry, 10.03.2020 00:52 jhonnysoriano9053
At 700 K, the reaction 2SO2(g) + O2(g) <> 2SO3(g) has the equilibrium constant Kc = 4.3 x 106. At a certain instant, following concentrations are present in the system: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
At 700 K, the reaction 2SO2(g) + O2(g) <> 2SO3(g) has the equilibrium constant Kc = 4.3 x 106....
Questions in other subjects:

Mathematics, 23.10.2020 06:01



Social Studies, 23.10.2020 06:01




Mathematics, 23.10.2020 06:01

Mathematics, 23.10.2020 06:01

Mathematics, 23.10.2020 06:01

![Q=\frac{[C]^{c} *[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0539/4103/8be7b.png)
![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0539/4103/e45ae.png)
![Q=\frac{[So_{3}] ^{2} }{[SO_{2} ]^{2}* [O_{2}] }](/tpl/images/0539/4103/dbc4a.png)