

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
A typical sample of vinegar has a pH of 3.0. Assuming that vinegar is only an aqueous solution of ac...
Questions in other subjects:





English, 03.10.2020 01:01

Mathematics, 03.10.2020 01:01





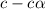


![pH=-log[H^+]](/tpl/images/0539/0805/15713.png)
![3.0=-log[H^+]](/tpl/images/0539/0805/d1dae.png)
![[H^+]=c\times \alpha=10^{-3}](/tpl/images/0539/0805/9452b.png)





