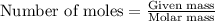Problem Page Question An analytical chemist weighs out of an unknown monoprotic acid into a volumetric flask and dilutes to the mark with distilled water. He then titrated this solution with solution. When the titration reaches the equivalence point, the chemist finds he has added of solution. Calculate the molar mass of the unknown acid. Round your answer to significant digits.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

You know the right answer?
Problem Page Question An analytical chemist weighs out of an unknown monoprotic acid into a volumetr...
Questions in other subjects:

English, 04.05.2021 03:40




Mathematics, 04.05.2021 03:40


English, 04.05.2021 03:40






 moles of HA
moles of HA