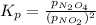Chemistry, 09.03.2020 20:03 dontcareanyonemo
A flask is charged with 1.500 atm of N2O4(g) and 1.00 atm NO2(g) at 25°C, and the following equilibrium is established. 2 NO2(g) ⇌ N2O4(g) At equilibrium, the partial pressure of NO2(g) is 0.512 atm. Calculate the equilibrium constant Kp for this reaction.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
A flask is charged with 1.500 atm of N2O4(g) and 1.00 atm NO2(g) at 25°C, and the following equilibr...
Questions in other subjects:

Chemistry, 22.03.2021 21:00

Mathematics, 22.03.2021 21:00


Social Studies, 22.03.2021 21:00


Mathematics, 22.03.2021 21:00

Mathematics, 22.03.2021 21:00

Social Studies, 22.03.2021 21:00

Health, 22.03.2021 21:00

 for the given reaction is 6.653
for the given reaction is 6.653