
Chemistry, 09.03.2020 18:50 lattimore12
Biphenyl, C12H10, is a nonvolatile, nonionizing solute that is soluble in benzene, C6H6. At 25 ∘C, the vapor pressure of pure benzene is 100.84 Torr. What is the vapor pressure of a solution made from dissolving 11.5 g of biphenyl in 31.9 g of benzene?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Biphenyl, C12H10, is a nonvolatile, nonionizing solute that is soluble in benzene, C6H6. At 25 ∘C, t...
Questions in other subjects:

Engineering, 11.12.2021 17:20



Chemistry, 11.12.2021 17:20

Mathematics, 11.12.2021 17:20

English, 11.12.2021 17:20

History, 11.12.2021 17:20



Mathematics, 11.12.2021 17:20


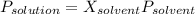 (1)
(1)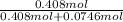 = 0.846
= 0.846


