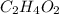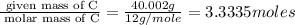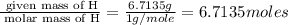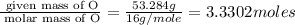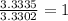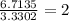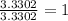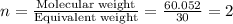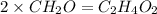Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
The percent composition by mass of an unknown compound with a molecular mass of 60.052 amu is 40.002...
Questions in other subjects:



Arts, 26.02.2021 16:30

Mathematics, 26.02.2021 16:30


Mathematics, 26.02.2021 16:30


Mathematics, 26.02.2021 16:30

Mathematics, 26.02.2021 16:30

English, 26.02.2021 16:30

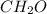 and
and