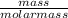What causes gas particles to collide with the walls of their containers?

Chemistry, 09.03.2020 06:46 stevencardona12mejia
Question 1.
What causes gas particles to collide with the walls of their containers?
A : Their volume
B : Their constant motion
C : Their pressure
D : Their small size
Question 2.
A balloon is filled with 5 grams of Helium gas. If the balloon takes up 10 mL of space and has a pressure of 20 mm Hg, which gas law needs to be used to find the temperature inside the balloon?
A: Charles law
B: Boyle’s law
C: Ideal gas law
D: Combined gas law

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Question 1.
What causes gas particles to collide with the walls of their containers?
What causes gas particles to collide with the walls of their containers?
Questions in other subjects:

Mathematics, 05.06.2020 18:03


Medicine, 05.06.2020 18:03



Biology, 05.06.2020 18:03

Chemistry, 05.06.2020 18:03

Mathematics, 05.06.2020 18:03