
Chemistry, 08.03.2020 21:11 genyjoannerubiera
C2 H4+ 3O2= 2CO2+2H2O how many moles of methane will be burnt to produce 0.1 of water

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
C2 H4+ 3O2= 2CO2+2H2O how many moles of methane will be burnt to produce 0.1 of water...
Questions in other subjects:

Mathematics, 08.02.2021 21:30






Physics, 08.02.2021 21:30


Biology, 08.02.2021 21:30

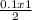 = 0.05 mole of C₂H₄
= 0.05 mole of C₂H₄


