
A sample of an ideal gas at 1.00 atm and a volume of 1.32 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 90.0 atm , what was the volume of the sample

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, tot92
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

You know the right answer?
A sample of an ideal gas at 1.00 atm and a volume of 1.32 L was placed in a weighted balloon and dro...
Questions in other subjects:

Mathematics, 17.01.2021 08:50


Arts, 17.01.2021 08:50


English, 17.01.2021 08:50

Mathematics, 17.01.2021 08:50

Mathematics, 17.01.2021 08:50

Mathematics, 17.01.2021 08:50

Mathematics, 17.01.2021 09:00

English, 17.01.2021 09:00

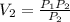 that is V₂ =
that is V₂ = 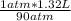 =
= 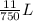 or 0.01467L
or 0.01467L

