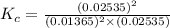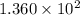Chemistry, 07.03.2020 05:42 jasminebrown72
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M . What is the equilibrium constant if the concentration of A at equilibrium is 0.01365 M ? 2A(aq)+B(aq)⇌2C(aq) Round your answer to one decimal place.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M ....
Questions in other subjects:

Mathematics, 09.09.2021 20:10







Physics, 09.09.2021 20:20


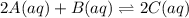
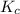 of the given reaction is as follows.
of the given reaction is as follows.![K_{c} = \frac{[C]^{2}}{[A]^{2}[B]}](/tpl/images/0538/0202/8ca5b.png)