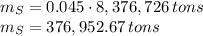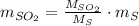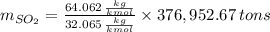Chemistry, 07.03.2020 05:01 qudoniselmore0
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 \rm tons of coal, a national record at that time.
Assuming that the coal was 86.0 \% carbon by mass and that combustion was complete, calculate the number of tons of carbon dioxide produced by the plant during the year.
Assuming that the coal was 4.50 \% sulfur by mass and that combustion was complete, calculate the number of tons of sulfur dioxide produced by the plant during the year.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1


Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 \rm tons of coal, a nat...
Questions in other subjects:







Mathematics, 03.07.2020 01:01

Chemistry, 03.07.2020 01:01


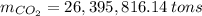 , b)
, b) 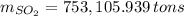
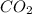 is produced by a mole of
is produced by a mole of 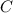 contained in coal. The yearly burnt carbon is:
contained in coal. The yearly burnt carbon is: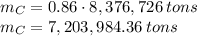
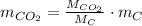
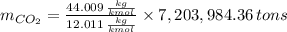
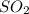 is produced by a mole of
is produced by a mole of 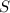 contained in coal.
contained in coal.