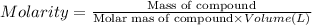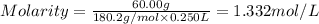Chemistry, 07.03.2020 04:59 forschoolok123456789
A student prepares a solution by dissolving 60.00 g of glucose (molar mass 180.2 g mol-1) in enough distilled water to make 250.0 mL of solution. The molarity of the solution should be reported as
a. 12.01 M
b. 12.0 M
c. 1.332 M
d. 1.33 M
e. 1.3 M

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
A student prepares a solution by dissolving 60.00 g of glucose (molar mass 180.2 g mol-1) in enough...
Questions in other subjects:

Mathematics, 29.01.2020 06:12



Mathematics, 29.01.2020 06:12

English, 29.01.2020 06:12

Mathematics, 29.01.2020 06:12

History, 29.01.2020 06:12


Business, 29.01.2020 06:12

History, 29.01.2020 06:12