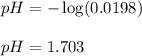Chemistry, 07.03.2020 05:25 suttonfae336
Monochloroacetic acid (HC2H2ClO2) is a skin irritant that is used in "chemical peels" intended to remove the top layer of dead skin from the face and ultimately improve the complexion. The value of Ka for monochloroacetic acid is 1.35 ✕ 10−3. Calculate the pH of a 0.31 M solution of monochloroacetic acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1


Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Monochloroacetic acid (HC2H2ClO2) is a skin irritant that is used in "chemical peels" intended to re...
Questions in other subjects:


Mathematics, 18.02.2021 19:10


History, 18.02.2021 19:10


Computers and Technology, 18.02.2021 19:10

Mathematics, 18.02.2021 19:10


Mathematics, 18.02.2021 19:10

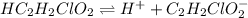
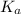 for above equation follows:
for above equation follows:![K_a=\frac{[H^+][C_2H_2ClO_2^-}}{[HC_2H_2ClO_2]}](/tpl/images/0537/9344/a55de.png)
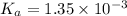
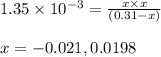
![pH=-\log[H^+]](/tpl/images/0537/9344/cf945.png)