
Chemistry, 07.03.2020 05:06 cnfndbxbfbdb2031
You have an evacuated container of fixed volume and known mass and introduce a known mass of a gas sample. measuring the pressure at constant temperature over time, you are surprised to see it slowly dropping. you measure the mass of the gas-filled container and find that the mass is what it should be - gas plus container - and the mass does not change over time, so you do not have a leak. part a suggest an explanation for your observations. suggest an explanation for your observations.
1. the gas undergoes a chemical reaction that has fewer gas particles in products than in reactants. pressure is directly proportional to number of particles, so the pressure decreased.
2. when the gas was plased into container it obtained some extra kinetic energy after some time this energy was expend, so the speed of the particles reduced and the pressure dropped the gas
3. undergoes a chemical reaction that has more gas particles in products than in reactants.
4. pressure is directly proportional to number of mass.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
You have an evacuated container of fixed volume and known mass and introduce a known mass of a gas s...
Questions in other subjects:


Mathematics, 08.10.2019 13:30


Chemistry, 08.10.2019 13:30



Mathematics, 08.10.2019 13:30

Mathematics, 08.10.2019 13:30


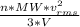
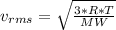 as such for pressure depends only on the number of moles so option 2 is cancelled out
as such for pressure depends only on the number of moles so option 2 is cancelled out

