

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2



Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
Arrange the metals used in this experiment (including H2 and Ag) in order of decreasing reactivity (...
Questions in other subjects:

Mathematics, 09.01.2021 01:30

Mathematics, 09.01.2021 01:30

Mathematics, 09.01.2021 01:30

History, 09.01.2021 01:30

English, 09.01.2021 01:30

Health, 09.01.2021 01:30



World Languages, 09.01.2021 01:30

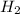 > Cu > Ag
> Cu > Ag

