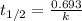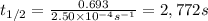Chemistry, 07.03.2020 04:45 Gghbhgy8716
The decomposition of N2O5 is described by the following equation. 2N2O5(g) → 4NO2(g) + O2(g) If the rate constant is 2.50 × 10−4 s−1, what is the half-life of this reaction?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 11:40, missmontgomery21
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2

Chemistry, 23.06.2019 14:30, Knownothing
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1

Chemistry, 23.06.2019 15:30, cristinaledford3696
Dona wrote the characteristics of two types of galaxies as shown below: type a: has a large flattened core type b: does not have a regular shape which statement is correct? type a is an irregular galaxy and type b is a lens galaxy. type a is a lens galaxy and type b is an irregular galaxy. type a is a spiral galaxy and type b is an elliptical galaxy. type a is an elliptical galaxy and type b is a spiral galaxy.
Answers: 2

Chemistry, 23.06.2019 20:30, nikkiebartels
What defines the mass number of an isotope? a. the sum of the neutrons and protons b. the sum of the neutrons and electrons c. the number of neutrons d. the number of protons
Answers: 3
You know the right answer?
The decomposition of N2O5 is described by the following equation. 2N2O5(g) → 4NO2(g) + O2(g) If the...
Questions in other subjects:

Mathematics, 28.08.2021 05:00




History, 28.08.2021 05:00

English, 28.08.2021 05:00

Biology, 28.08.2021 05:00



English, 28.08.2021 05:00

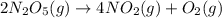
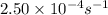
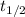 ) and rate constant (k)are related by :
) and rate constant (k)are related by :