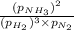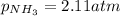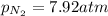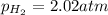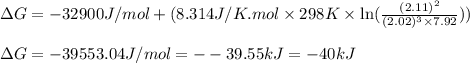Chemistry, 07.03.2020 04:48 Mattisback2285
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2.11 atm ammonia (NH3) gas at a temperature of 25.0°C
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction
N2(8) +3H2 2NH3 (g)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1


You know the right answer?
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2...
Questions in other subjects:


Chemistry, 15.04.2020 23:10


Biology, 15.04.2020 23:10





Mathematics, 15.04.2020 23:10

Mathematics, 15.04.2020 23:10

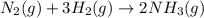
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(NH_3(g))})]-[(1\times \Delta G^o_f_{(N_2(g))})+(3\times \Delta G^o_f_{(H_2(g))})]](/tpl/images/0537/7676/6e73c.png)
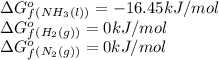
![\Delta G^o_{rxn}=[(2\times (-16.45))]-[(1\times (0))+(3\times (0))]\\\\\Delta G^o_{rxn}=-32.9kJ/mol](/tpl/images/0537/7676/8bdb6.png)
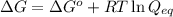
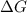 = free energy of the reaction
= free energy of the reaction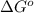 = standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0537/7676/0e82f.png)
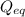 = Ratio of concentration of products and reactants at any time =
= Ratio of concentration of products and reactants at any time =