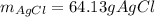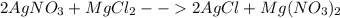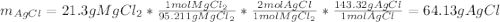Chemistry, 07.03.2020 04:49 izaiahfieods
When silver nitrate is added to an aqueous solution of magnesium chloride, a precipitation reaction occurs that produces silver chloride and magnesium nitrate. When enough AgNO3 is added so that 21.3 g of MgCl2 react, what mass of the AgCl precipitate should form

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, ezrasedore
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1


Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2
You know the right answer?
When silver nitrate is added to an aqueous solution of magnesium chloride, a precipitation reaction...
Questions in other subjects:



Mathematics, 24.09.2021 19:50



English, 24.09.2021 19:50

Business, 24.09.2021 19:50


Mathematics, 24.09.2021 19:50

Mathematics, 24.09.2021 19:50