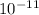Chemistry, 07.03.2020 04:02 hannahpalacios101
. Kc = 2.19*10-10 @ 100 oC for the rxn: COCl2(g) CO(g) + Cl2(g) the following mixtures may or may not be at equilibrium. Identify which mixtures are at equilibrium, if not, indicate which direction the reaction will go to obtain equilibrium (don’t guess, no credit given without supporting evidence). a) [CO] = 1.0*10-3, [Cl2] = 1.0*10-3, [COCl2]= 2.19*10-1 b) [CO] = 3.31*10-6, [Cl2] = 3.31*10-6, [COCl2]= 5.00*10-2 c) [CO] = 4.5*10-7, [Cl2] = 5.73*10-6, [COCl2]= 8.57*10-2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
You know the right answer?
. Kc = 2.19*10-10 @ 100 oC for the rxn: COCl2(g) CO(g) + Cl2(g) the following mixtures may or may...
Questions in other subjects:

Mathematics, 20.09.2020 08:01



Mathematics, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01





![\frac{[CO][Cl2]}{[COCl2]}](/tpl/images/0537/5342/fdabd.png)
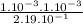 = 0.456.
= 0.456.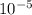
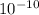 ), it can be deducted that it is not in equilibrium, since Kc1≠Kc and Kc1 is larger than Kc. When that occurs, it means the reaction is favoring the products, producing more of it. So, the equilibrium is going to the right.
), it can be deducted that it is not in equilibrium, since Kc1≠Kc and Kc1 is larger than Kc. When that occurs, it means the reaction is favoring the products, producing more of it. So, the equilibrium is going to the right.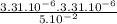 = 0.662.
= 0.662.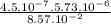 = 3.01.
= 3.01.