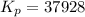Chemistry, 07.03.2020 03:29 tashakelly42
Consider the following reaction N2(g) + 3H2(g) 2NH3(g)0.866 atm of N2 and 0.0496 atm of H2 are placed in a flask; when equilibrium is reached, the pressure of NH3 is 0.0310 atm. Calculate Kpfor the reaction. Use the following steps to solvethis problem:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


You know the right answer?
Consider the following reaction N2(g) + 3H2(g) 2NH3(g)0.866 atm of N2 and 0.0496 atm of H2 are place...
Questions in other subjects:

English, 15.04.2020 21:55


Mathematics, 15.04.2020 21:55


Physics, 15.04.2020 21:55

Mathematics, 15.04.2020 21:55


Mathematics, 15.04.2020 21:55


Mathematics, 15.04.2020 21:55

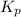 is 37928.
is 37928.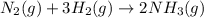
![K_p=\frac{[p_{NH_3}]^2}{p_{N_2}\times [p_{H_2}]^3}](/tpl/images/0537/3920/ee3ee.png)
![K_p=\frac{[2x]^2}{(0.866-x)\times (0.0496-3x)^3}](/tpl/images/0537/3920/b2858.png)