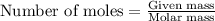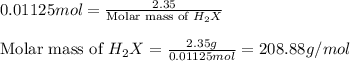Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, Mercedes12152002
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1


Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
A 2.35-g sample of an acid, H2X, requires 45.0 mL of a 0.500 M NaOH solution for complete reaction (...
Questions in other subjects:



History, 23.08.2019 04:30

Chemistry, 23.08.2019 04:30

Biology, 23.08.2019 04:30

Biology, 23.08.2019 04:30

English, 23.08.2019 04:30


Mathematics, 23.08.2019 04:30

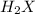 is 208.88 g/mol
is 208.88 g/mol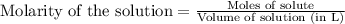
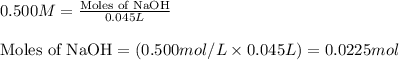
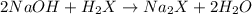
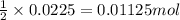 of
of