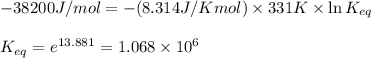Chemistry, 07.03.2020 03:46 Joshuafranklindude
In the Mond process for the purification of nickel, carbon monoxide is reacted with heated nickel to produce Ni(CO)4, which is a gas and can therefore be separated from solid impurities: Ni(s) + 4CO(g) ⇌ Ni(CO)4(g) Given that the standard free energies of formation of CO(g) and Ni(CO)4(g) are −137.3 and −587.4 kJ/mol, respectively, calculate the equilibrium constant of the reaction at 58.0°C. Assume that ΔG o f is temperature-independent.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, mahoganyking16
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2



Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
In the Mond process for the purification of nickel, carbon monoxide is reacted with heated nickel to...
Questions in other subjects:

Mathematics, 31.08.2021 21:20

Mathematics, 31.08.2021 21:20

Mathematics, 31.08.2021 21:20


Mathematics, 31.08.2021 21:30

Health, 31.08.2021 21:30

Mathematics, 31.08.2021 21:30

Computers and Technology, 31.08.2021 21:30

Mathematics, 31.08.2021 21:30

English, 31.08.2021 21:30

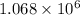
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_{(product)}]-\sum [n\times \Delta G^o_{(reactant)}]](/tpl/images/0537/4791/f0852.png)
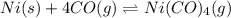
![\Delta G^o_{rxn}=[(1\times \Delta G^o_{(Ni(CO)_4(g))})]-[(1\times \Delta G^o_{(Ni(s))})+(4\times \Delta G^o_{(CO(g))})]](/tpl/images/0537/4791/5f698.png)
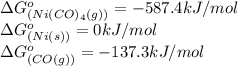
![\Delta G^o_{rxn}=[(1\times (-587.4))]-[(1\times (0))+(4\times (-137.3))]\\\\\Delta G^o_{rxn}=-38.2kJ/mol](/tpl/images/0537/4791/37589.png)
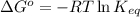
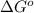 = Standard Gibbs free energy = -38.2 kJ/mol = -38200 J/mol (Conversion factor: 1 kJ = 1000 J )
= Standard Gibbs free energy = -38.2 kJ/mol = -38200 J/mol (Conversion factor: 1 kJ = 1000 J )![58^oC=[273+58]K=331K](/tpl/images/0537/4791/2a33f.png)
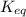 = equilibrium constant at 58°C = ?
= equilibrium constant at 58°C = ?