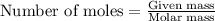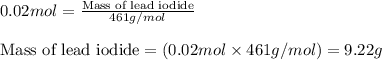Chemistry, 07.03.2020 02:57 graymonky12
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include phases. chemical equation: What mass of precipitate will form if 1.50 L of highly concentrated Pb ( ClO 3 ) 2 is mixed with 0.200 L 0.200 M NaI ? Assume the reaction goes to completion.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, Tyrant4life
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include ph...
Questions in other subjects:


Social Studies, 12.04.2021 15:30

History, 12.04.2021 15:30



Health, 12.04.2021 15:30


Mathematics, 12.04.2021 15:30


English, 12.04.2021 15:30

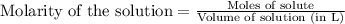
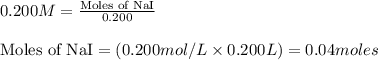
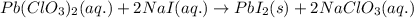
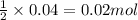 of lead iodide
of lead iodide