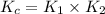Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium reaction arrow 1/4 P4(g) + 5/2 Cl2(g); Kc = 1.18 ✕ 10−21 1/4 P4(g) + 3/2 Cl2(g) equilibrium reaction arrow PCl3(g); Kc = 2.28 ✕ 1026 Calculate Kc for PCl5(g) equilibrium reaction arrow PCl3(g) + Cl2(g) at the same temperature.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jalenshayewilliams
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium react...
Questions in other subjects:






Physics, 16.09.2021 14:10

English, 16.09.2021 14:10

English, 16.09.2021 14:10

Mathematics, 16.09.2021 14:10

English, 16.09.2021 14:10

 for the net reaction is
for the net reaction is 
![PCl_5(g)\xrightarrow[]{K_1} \frac{1}{4}P_4(g)+\frac{5}{2}Cl_2(g)](/tpl/images/0537/3407/f38c5.png)
![\frac{1}{4}P_4(g)+\frac{3}{2}Cl_2(g)\xrightarrow[]{K_2} PCl_5(g)](/tpl/images/0537/3407/948a1.png)
![PCl_5(g)\xrightarrow[]{K_c} PCl_3(g)+Cl_2(g)](/tpl/images/0537/3407/3c32f.png)