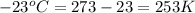Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 04:40, laurabwhiddon
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 05:30, hongkongbrat6840
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2

Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
A helium balloon with an internal pressure of 1.0 atm and a volume of 4.50 L at 20.0⁰C is released....
Questions in other subjects:


Computers and Technology, 04.01.2021 03:20

Mathematics, 04.01.2021 03:20

SAT, 04.01.2021 03:20





Spanish, 04.01.2021 03:20

Mathematics, 04.01.2021 03:20


 = initial pressure of gas = 1 atm = 101.3 kPa
= initial pressure of gas = 1 atm = 101.3 kPa = final pressure of gas = 60.78 kPa
= final pressure of gas = 60.78 kPa = initial volume of gas = 4.50 L
= initial volume of gas = 4.50 L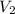 = final volume of gas = ?
= final volume of gas = ?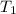 = initial temperature of gas =
= initial temperature of gas = 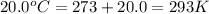
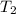 = final temperature of gas =
= final temperature of gas =