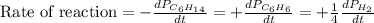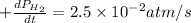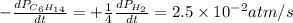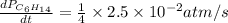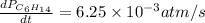Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1
You know the right answer?
For the reaction C6H14(g) > C6H6(g) + 4H2(g), the rate of formation of hydrogen gas, H2 was found...
Questions in other subjects:










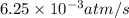
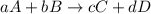
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0537/1900/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0537/1900/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0537/1900/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0537/1900/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0537/1900/d4b94.png)
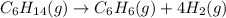
![\text{Rate of disappearance of }C_6H_{14}=-\frac{d[C_6H_{14}]}{dt}](/tpl/images/0537/1900/89957.png)
![\text{Rate of formation of }C_6H_6=+\frac{d[C_6H_6]}{dt}](/tpl/images/0537/1900/d8137.png)
![\text{Rate of formation of }H_2=+\frac{1}{4}\frac{d[H_2]}{dt}](/tpl/images/0537/1900/ecdf8.png)