
Chemistry, 07.03.2020 02:48 gizmo50245
He rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy . If the rate constant of this reaction is at , what will the rate constant be at ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2



Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
He rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activ...
Questions in other subjects:




Biology, 07.08.2021 02:00

Mathematics, 07.08.2021 02:00

Biology, 07.08.2021 02:00




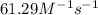
![\ln(\frac{K_{324^oC}}{K_{244^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0537/2208/1ff41.png)
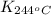 = equilibrium constant at 244°C =
= equilibrium constant at 244°C = 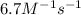
 = equilibrium constant at 324°C = ?
= equilibrium constant at 324°C = ?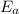 = Activation energy = 71.0 kJ/mol = 71000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 71.0 kJ/mol = 71000 J/mol (Conversion factor: 1 kJ = 1000 J)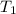 = initial temperature =
= initial temperature = ![244^oC=[273+244]K=517K](/tpl/images/0537/2208/c11e6.png)
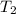 = final temperature =
= final temperature = ![324^oC=[273+324]K=597K](/tpl/images/0537/2208/4d511.png)
![\ln(\frac{K_{324^oC}}{6.7})=\frac{71000J}{8.314J/mol.K}[\frac{1}{517}-\frac{1}{597}]\\\\K_{324^oC}=61.29M^{-1}s^{-1}](/tpl/images/0537/2208/108e2.png)



