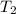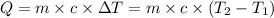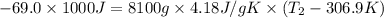A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8.10kg of water at 33.9 degrees celsius . During the reaction 69.0kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J*g*K. Round your answer to 3 significant digits.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8....
Questions in other subjects:



Mathematics, 10.02.2020 10:37

Mathematics, 10.02.2020 10:37



Mathematics, 10.02.2020 10:37


English, 10.02.2020 10:38

Mathematics, 10.02.2020 10:38