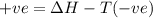Chemistry, 07.03.2020 01:44 kayranicole1
Which of the following indicates a reaction with a positive ΔG? A. endergonic, spontaneous B. endergonic, not spontaneous C. exergonic, spontaneous D. exergonic, not spontaneous

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Which of the following indicates a reaction with a positive ΔG? A. endergonic, spontaneous B. enderg...
Questions in other subjects:


Mathematics, 02.08.2019 08:50

Mathematics, 02.08.2019 08:50


Mathematics, 02.08.2019 08:50

History, 02.08.2019 08:50

Mathematics, 02.08.2019 08:50


English, 02.08.2019 08:50

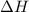 for the reaction comes out to be positive.
for the reaction comes out to be positive.
 = Gibb's free energy change
= Gibb's free energy change = entropy change
= entropy change