

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an acti...
Questions in other subjects:

Mathematics, 07.05.2021 18:50

Mathematics, 07.05.2021 18:50

History, 07.05.2021 18:50




Medicine, 07.05.2021 18:50

Mathematics, 07.05.2021 18:50


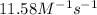
![\ln(\frac{K_{56.0^oC}}{K_{-8.0^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0536/9043/5e447.png)
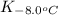 = equilibrium constant at -8.0°C =
= equilibrium constant at -8.0°C = 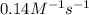
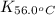 = equilibrium constant at 56°C = ?
= equilibrium constant at 56°C = ?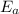 = Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)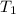 = initial temperature =
= initial temperature = ![-8.0^oC=[273-8]K=265K](/tpl/images/0536/9043/f4197.png)
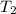 = final temperature =
= final temperature = ![56.0^oC=[273+56]K=329K](/tpl/images/0536/9043/23b22.png)
![\ln(\frac{K_{56.0^oC}}{0.14})=\frac{50000J}{8.314J/mol.K}[\frac{1}{265}-\frac{1}{329}]\\\\K_{56.0^oC}=11.58M^{-1}s^{-1}](/tpl/images/0536/9043/35497.png)



