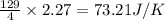Chemistry, 07.03.2020 00:46 flowerrbabie
Consider the reaction 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.27 moles of HCl(g) react at standard conditions. Ssurroundings = J/K

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Consider the reaction 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard thermodynamic data at 298K, ca...
Questions in other subjects:

Biology, 08.01.2020 15:31

Mathematics, 08.01.2020 15:31

Mathematics, 08.01.2020 15:31


Mathematics, 08.01.2020 15:31

Mathematics, 08.01.2020 15:31


Mathematics, 08.01.2020 15:31


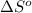 for the surrounding when given amount of HCl gas is reacted is 73.21 J/K
for the surrounding when given amount of HCl gas is reacted is 73.21 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0536/8507/52737.png)
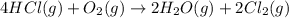
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(Cl_2(g))})+(2\times \Delta S^o_{(H_2O(g))})]-[(4\times \Delta S^o_{(HCl(g))})+(1\times \Delta S^o_{(O_2(g))})]](/tpl/images/0536/8507/2475f.png)
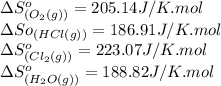
![\Delta S^o_{rxn}=[(2\times (223.07))+(2\times (188.82))]-[(4\times (186.91))+(1\times (205.14))]\\\\\Delta S^o_{rxn}=-129J/K](/tpl/images/0536/8507/8e27e.png)