
Chemistry, 07.03.2020 00:59 ElegantEmerald
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chloride.
The complete ionic equation contains which of the following species (when balanced in standard form)?
a.
Cl-(aq)
b.
2Na+(aq)
c.
2S2-(aq)
d.
Na+(aq)
e.
3Mg2+(aq)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

You know the right answer?
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chlo...
Questions in other subjects:


Business, 30.11.2021 21:40

Mathematics, 30.11.2021 21:40






Mathematics, 30.11.2021 21:40

Mathematics, 30.11.2021 21:40

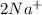
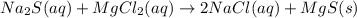
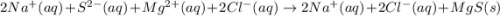
 are the spectator ions.
are the spectator ions.

