
Chemistry, 07.03.2020 00:34 Candieboo4006
Ammonium hydrogen sulfide decomposes according to the following reaction, for which Kp = 0.11 at 250ºC: NH4HS(s) H2S(g) + NH3(g) If 55.0 g of NH4HS(s) is placed in a sealed 5.0-L container, what is the partial pressure of NH3(g) at equilibrium

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Ammonium hydrogen sulfide decomposes according to the following reaction, for which Kp = 0.11 at 250...
Questions in other subjects:


Spanish, 08.12.2020 18:40


Business, 08.12.2020 18:40

Biology, 08.12.2020 18:40




English, 08.12.2020 18:40

English, 08.12.2020 18:40

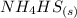 ⇄
⇄ 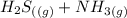
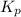 = 0.11
= 0.11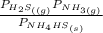
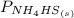 = 1 since it is solid and solid and it is known that any solid has uniformity at equilibrium.
= 1 since it is solid and solid and it is known that any solid has uniformity at equilibrium.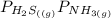
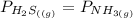
![[x][x]](/tpl/images/0536/8008/ce5d5.png)
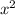
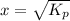
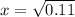
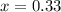 atm
atm

