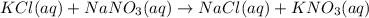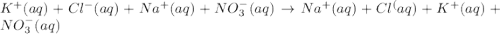Chemistry, 07.03.2020 00:02 raidattarab
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The complete ionic equation contains which of the following species (when balanced in standard form)?
a. Cl-(aq)
b. KNO3(aq)
c.3NO-3(aq)
d.2Na+(aq)
e.2K+(aq)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1


Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. The c...
Questions in other subjects:

Spanish, 27.10.2019 11:43



History, 27.10.2019 11:43


Mathematics, 27.10.2019 11:43

Biology, 27.10.2019 11:43



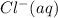 ions are present in the complete ionic equation
ions are present in the complete ionic equation