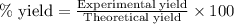Chemistry, 07.03.2020 00:10 peperivera2652738
A student measures out exactly 0.105 g of salicylic acid and runs the experiment as dictated in the lab manual. They obtain 0.111 g of aspirin. What is the percent yield for their reaction?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 08:50, leah5981
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas. caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible. relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
A student measures out exactly 0.105 g of salicylic acid and runs the experiment as dictated in the...
Questions in other subjects:




History, 22.04.2021 16:10

History, 22.04.2021 16:10




French, 22.04.2021 16:10

Geography, 22.04.2021 16:10

 .....(1)
.....(1)

 of aspirin
of aspirin