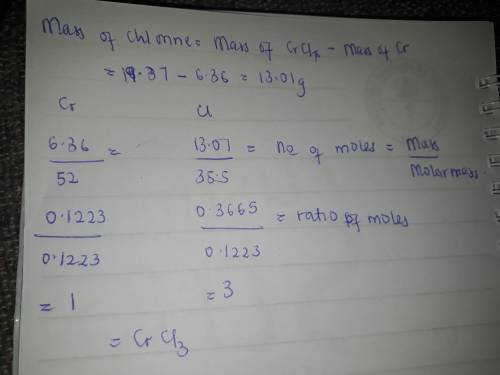Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
When 6.36 g of metallic chromium is heated with elemental chlorine gas, 19.37 g of a chromium chlori...
Questions in other subjects:

Mathematics, 01.01.2020 04:31




Mathematics, 01.01.2020 04:31



Mathematics, 01.01.2020 04:31

Mathematics, 01.01.2020 04:31