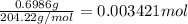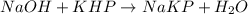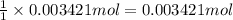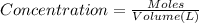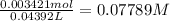Chemistry, 07.03.2020 00:21 fdougie111
A NaOH solution is standardized using the monoprotic primary standard potassium hydrogen phthalate, KHP (204.22 g/mol.) If 0.6986 g of KHP requires 43.92 mL of NaOH, what is the NaOH concentration

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 08:00, vetterk1400
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
A NaOH solution is standardized using the monoprotic primary standard potassium hydrogen phthalate,...
Questions in other subjects:


Biology, 10.12.2021 03:50



Mathematics, 10.12.2021 03:50


Geography, 10.12.2021 03:50


English, 10.12.2021 03:50