
Chemistry, 06.03.2020 08:52 Nyasiabaltimore3
"To determine the amount of heroin in the mixture, you dissolve 1.00 g of the white powdery mixture in water in a 100.0-mL volumetric flask. You find that the solution has an osmotic pressure of 531 mm Hg at 25 °C. What is the composition of the mixture?"

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1


Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
"To determine the amount of heroin in the mixture, you dissolve 1.00 g of the white powdery mixture...
Questions in other subjects:

Physics, 14.12.2020 23:30


Social Studies, 14.12.2020 23:30





Mathematics, 14.12.2020 23:30


Mathematics, 14.12.2020 23:30

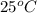
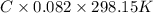
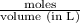 = 0.0285
= 0.0285
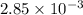
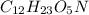 = x grams
= x grams
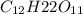 = (1.00 - x)
= (1.00 - x)
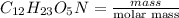
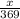
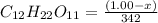
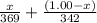
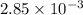
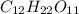 present is (1 - 0.346) g = 0.654 g.
present is (1 - 0.346) g = 0.654 g.

