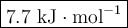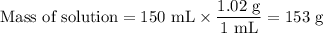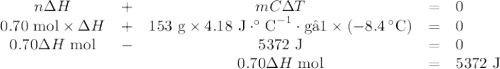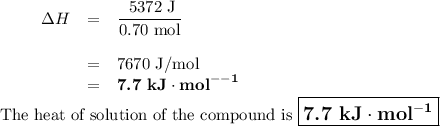0.70 moles of an unknown solid is placed into water to make 150.0 mL of solution. The solution's temperature decreases by 8.4°C. Calculate ∆H for the dissolution of the unknown solid. (The specific heat of the solution is 4.18 J/g・°C and the density of the solution is 1.02 g/mL).

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, YatesDevon3371
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1


Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
0.70 moles of an unknown solid is placed into water to make 150.0 mL of solution. The solution's tem...
Questions in other subjects:

History, 03.03.2021 06:20





Computers and Technology, 03.03.2021 06:20

Mathematics, 03.03.2021 06:20



Mathematics, 03.03.2021 06:20