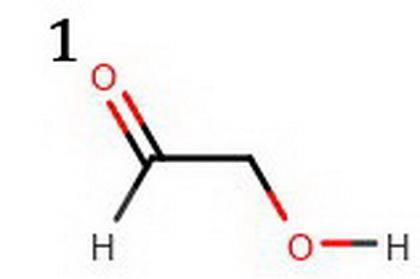
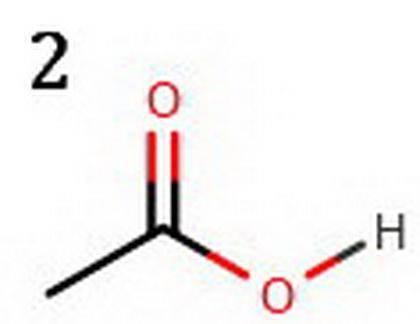
The following two compounds are constitutional isomers. Identify which of these is expected to be more acidic, and explain your choice. a. The compound below is more acidic because its conjugate base is more resonance stabilized. b. The conjugate base of the other compound is not as much resonance stabilized. c. The compound below is more acidic because its conjugate base is more resonance stabilized. d. The conjugate base of the other compound is not as much resonance stabilized. e. The compound below is more acidic because its conjugate base is resonance stabilized. f. The conjugate base of the other compound is not resonance stabilized. Tg. he compound below is more acidic because its conjugate base is resonance stabilized. h. The conjugate base of the other compound is not resonance stabilized.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
The following two compounds are constitutional isomers. Identify which of these is expected to be mo...
Questions in other subjects:

Mathematics, 25.09.2020 22:01

Mathematics, 25.09.2020 22:01



Law, 25.09.2020 22:01


Physics, 25.09.2020 22:01

Mathematics, 25.09.2020 22:01

Geography, 25.09.2020 22:01