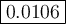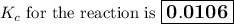Calculate the value of the equilibrium constant, K c , for the reaction Q ( g ) + X ( g ) − ⇀ ↽ − 2 M ( g ) + N ( g ) given that M ( g ) − ⇀ ↽ − Z ( g ) K c 1 = 3.15 6 R ( g ) − ⇀ ↽ − 2 N ( g ) + 4 Z ( g ) K c 2 = 0.509 3 X ( g ) + 3 Q ( g ) − ⇀ ↽ − 9 R ( g ) K c 3 = 12.5

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Calculate the value of the equilibrium constant, K c , for the reaction Q ( g ) + X ( g ) − ⇀ ↽ − 2...
Questions in other subjects:



Mathematics, 12.10.2019 22:00



Mathematics, 12.10.2019 22:00

Geography, 12.10.2019 22:00



History, 12.10.2019 22:00