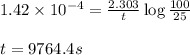Chemistry, 06.03.2020 01:31 xitlalizt83341
The decomposition of SO2Cl2 is first order in SO2Cl2 and has a rate constant of 1.42 x 10-4 s-1 at a certain temperature. How long will it take for the concentration of SO2Cl2 to decrease to 25% of its initial concentration

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

You know the right answer?
The decomposition of SO2Cl2 is first order in SO2Cl2 and has a rate constant of 1.42 x 10-4 s-1 at a...
Questions in other subjects:

Geography, 23.01.2021 02:10

English, 23.01.2021 02:10

English, 23.01.2021 02:10

Social Studies, 23.01.2021 02:10


Mathematics, 23.01.2021 02:10



English, 23.01.2021 02:10

![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0534/9883/f1041.png)
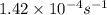
![[A_o]](/tpl/images/0534/9883/dc622.png) = initial amount of the reactant = 100 grams
= initial amount of the reactant = 100 grams