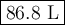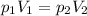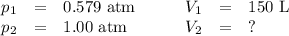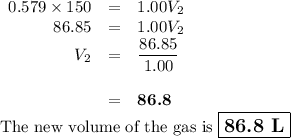Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
You know the right answer?
A pump contains 1.5 L of air at 175 kPa. You draw back on the piston of the pump, expanding the volu...
Questions in other subjects:

Mathematics, 01.03.2021 18:40


Mathematics, 01.03.2021 18:40


Mathematics, 01.03.2021 18:40


Arts, 01.03.2021 18:40


Mathematics, 01.03.2021 18:40