
Chemistry, 05.03.2020 03:40 joThompson
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentrations are found to be [PCl5] = 0.40 M, [PCl3] = [Cl2] = 0.20. If the volume of the container is suddenly halved at the same temperature, what will be the new equilibrium concentration of PCl5?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 05:50, cicimarie2018
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentr...
Questions in other subjects:

Biology, 28.10.2020 18:40

Mathematics, 28.10.2020 18:40

Chemistry, 28.10.2020 18:40

Mathematics, 28.10.2020 18:40

History, 28.10.2020 18:40


Mathematics, 28.10.2020 18:40

Mathematics, 28.10.2020 18:40

Health, 28.10.2020 18:40

Mathematics, 28.10.2020 18:40

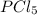 will be 0.9 M.
will be 0.9 M.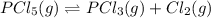
![[PCl_5]=0.40 M](/tpl/images/0534/1154/cb9c6.png)
![[PCl_3]=[Cl_2]=0.20 M](/tpl/images/0534/1154/9bc6a.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0534/1154/73fe0.png)
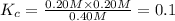
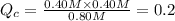
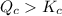
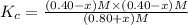
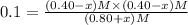
![[PCl_5]=(0.80+x) M=(0.80+0.1) M = 0.90](/tpl/images/0534/1154/dbad2.png)


