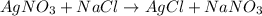Chemistry, 05.03.2020 00:22 tytybruce2
On a balance, you have beakers of AgNO3AgNO3 solution and NaClNaCl solution. When mixed, they will form AgCl(s)AgCl(s). What will happen to the mass of the new mixture?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
On a balance, you have beakers of AgNO3AgNO3 solution and NaClNaCl solution. When mixed, they will f...
Questions in other subjects:

Chemistry, 07.04.2021 16:40

Mathematics, 07.04.2021 16:40


Spanish, 07.04.2021 16:40





History, 07.04.2021 16:40

 is added to NaCl then the compound formed will have same mass as that of reactants.
is added to NaCl then the compound formed will have same mass as that of reactants.