
Chemistry, 04.03.2020 23:26 TheOneandOnly003
A compound is 92.2% Carbon and 7.76% Hydrogen. The formula mass of the compound is 78.1 g. Calculate the empirical formula and molecular formula of the compound.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

You know the right answer?
A compound is 92.2% Carbon and 7.76% Hydrogen. The formula mass of the compound is 78.1 g. Calculate...
Questions in other subjects:



Mathematics, 17.10.2020 23:01

History, 17.10.2020 23:01

English, 17.10.2020 23:01


Geography, 17.10.2020 23:01

Mathematics, 17.10.2020 23:01


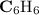

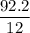
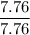
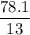
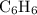 .
.


